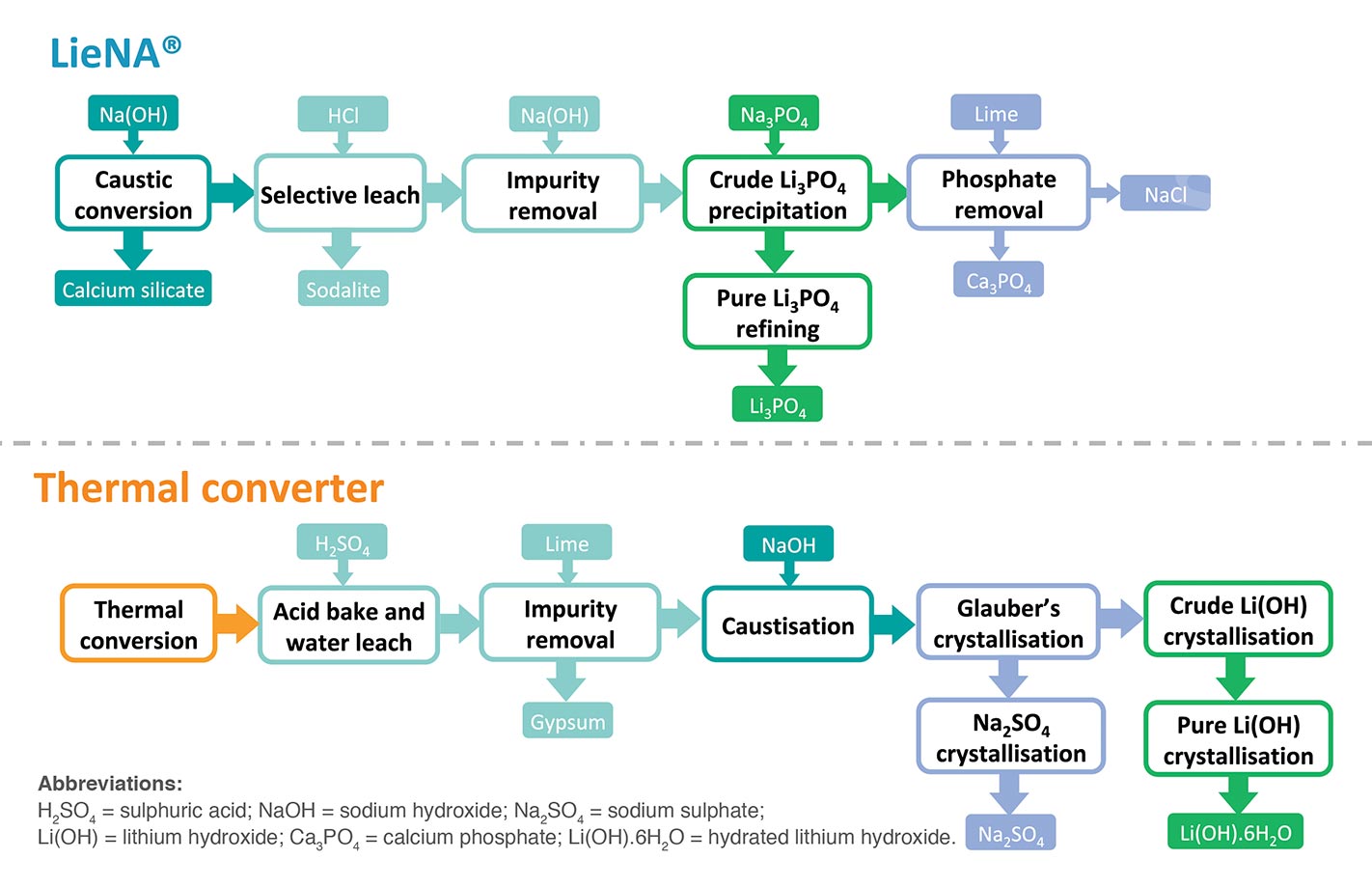
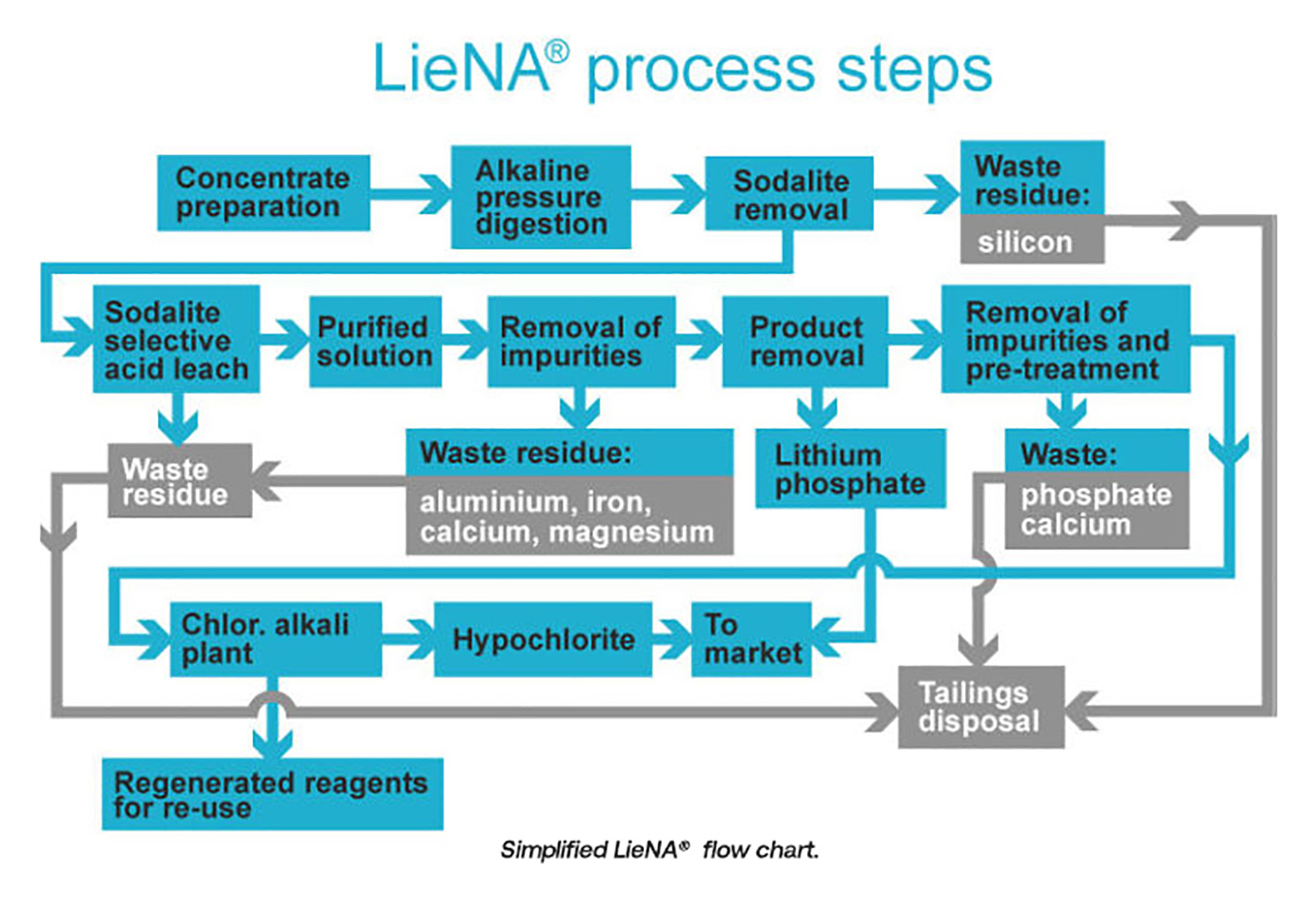
Historically, lithium was mined from pegmatite deposits in North Carolina, Zimbabwe, Portugal, Brazil and China. With the advent of cheaper production of lithium from brines in South America (and to a lesser extent China) in the 1980s, hard-rock production in the above-mentioned countries dwindled. From the 1990s on, production of spodumene concentrate came to be dominated by the Greenbushes mine in Western Australia, a situation that persisted until the commissioning of new production in that state by the likes of Galaxy, Pilbara Minerals, Altura, Minres, Neometals, etc.
More recent developments in Western Australia (Wadgina, Pilgangora, Mt Marion, Bald Hill, Mt Cattlin and Earl Grey, as well as the expansion of Greenbushes) will result in considerable extra feed, as will new projects in Canada and southern Africa, but requirements are still likely to outstrip supply longer term.
Spodumene deposits mined to date have shown yields to commercial lithium concentrate of 50-70%, with some operations targeting optimised recoveries of 75%. But where is the rest reporting?
Lithium pegmatites are often characterised by complex mineralogy, with the lithium occurring in more than one mineral phase. Product specifications for commercial spodumene concentrates preclude other lithium mineral contaminants, meaning that lithium micas, petalite, amblygonite, eucryptite, etc. are rejected as tailings. Under some circumstances this can result in lithium losses as high as 30%.